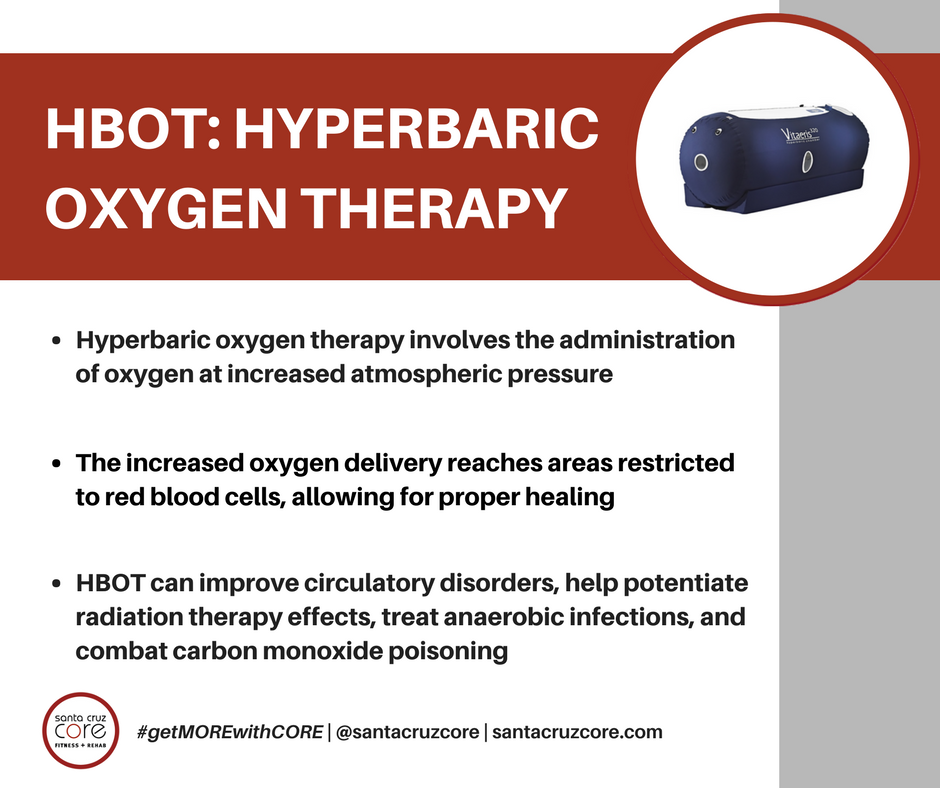
Hyperbaric oxygen therapy (HBOT) treats a variety of medical conditions ranging from bacterial infections to arterial air embolisms.

The use of hyperbaric oxygen has had increasing positive outcomes in clinical practice. Physicians are becoming continually more aware of its therapeutic mechanisms.
HBOT also prolongs safe anesthesia and circulatory arrest during surgery (Gill, 2004). Hospitals typically have a hyperbaric oxygen room or chamber, used in cases such as decompression sickness and carbon monoxide poisoning. However, its therapeutic use is much broader. HBOT can improve the state of circulatory disorders, help potentiate radiation th
erapy effects, treat anaerobic infections, and combat carbon monoxide poisoning.
What is HBOT?
Hyperbaric oxygen therapy (HBOT) involves the administration of 100% oxygen at increased atmospheric pressure. Normally, we breathe gases in the air (including oxygen) at the normal atmospheric pressure of 1 atmosphere (1 ATA). During HBOT, a high amount of oxygen is administered and atmospheric pressure is increased (typically to 3 ATA). The treatment is systemic and controlled, the process developed over years of study and practice.
How does HBOT work?
The benefits of hyperbaric oxygen can be verified by understanding gas laws and how oxygen is delivered to tissues.
Most oxygen is delivered to tissues via hemoglobin which is found in red blood cells. At the normal atmospheric pressure (1 ATA), oxygen saturates about 97% of hemoglobin. However, some oxygen is also carried via solution (blood plasma). Increasing pressure means increasing the amount of oxygen delivered by the solution.
The Gas Laws
Boyle’s Law states the inverse proportional relationship between gas volume and gas pressure at constant temperature. This means more pressure means less volume and less pressure means more volume. This same gas relationship makes decompression of gas dangerous when trapped in the body, such as in circulation.
Dolton’s Law then brings in the concept of the partial pressure of gases into the mix. Partial pressure refers to the pressure amount of a single type of gas (such as oxygen), which contributes to the overall pressure of a mixture of gases (such as air). Therefore, the pressure contributed by a single type of gas is proportional to its volume. So, more partial pressure equals more volume and vice versa.
Henry’s Law then states that the amount of gas which dissolves into a liquid or tissue is proportionally dependent on the partial pressure of that gas.
With these facts about tissue oxygen delivery and gas laws in mind, one can begin to understand how HBOT works. The increased oxygen delivery via solution reaches areas restricted to red blood cells, allowing for proper healing. Oxygen in solution under these conditions is enough to support resting tissues without red blood cells. This also allows oxygen delivery despite the presence of conditions that limit the effectiveness of red blood cells.
References:
Gill, A.L, and Bell. “Hyperbaric Oxygen: Its Uses, Mechanisms of Action and Outcomes | QJM: An International Journal of Medicine | Oxford Academic.” OUP Academic, Oxford University Press, 1 July 2004, academic.oup.com/qjmed/article/97/7/385/1605756/Hyperbaric-oxygen-its-uses-mechanisms-of-action.
Would you like to learn more about Hyperbaric Oxygen Therapy? Come to CORE and we’ll tell you all about it! We’ve recently introduced our own Hyperbaric Oxygen Chamber operated by our on-site D.O., Dr. John Grady. Our intro offer includes 2 Hyperbaric Therapy sessions for $198! 👇








Your blood carries this oxygen throughout your body. This helps fight bacteria and stimulate the release of substances called growth factors and stem cells, which promote healing.
Also very true! Thank you 3movs.